Cuban vaccine Soberana-02 ready for phase 3 clinical trials
Cuba may be the only Latin American country to produce its own Covid-19 vaccine.
- Información
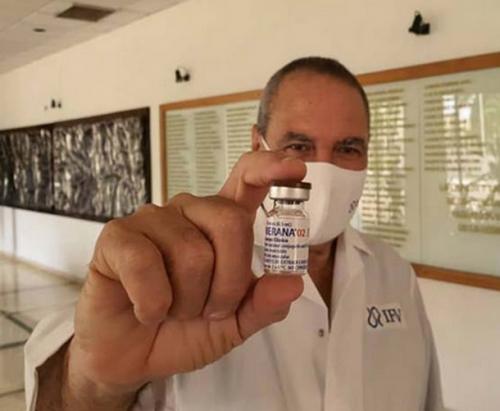
Cuba's Covid-19 vaccine, Soberana 02, is ready to begin phase 03 (final phase) clinical testing with some 44,000 volunteers next week, the Finlay Institute announced today.
During a meeting with foreign and domestic media, the entity's director, Vicente Vérez, showed the vial with the product for the start of this stage of testing in Havana municipalities.
"The reality of a dream, Soberana 02 ready for the start of the phase 3 clinical trial in several municipalities of Havana. Another milestone of Cuban science in the era of Covid-19. The pride of us all...”, states a message posted on the Finlay Institute's official Twitter account.
Vérez recently confirmed that the country is also preparing for the production of 100 million doses of the vaccine, with which all foreigners interested in the Cuban product can be immunized.
The meeting with the media was attended by officials from the Center for Molecular Immunology (CIM), in whose factories the production of the RBD antigen, for Soberana 02, is being scaled up.
The director general of the CIM, Eduardo Ojito, pointed out that the capabilities and productive potential of this entity has allowed them to produce so far the 340 thousand doses of the candidate vaccine for this new stage.
The day before, the Biotechnology and Pharmaceutical Industries Business Group of the Caribbean country also announced the large-scale production of another vaccine, called Abdala, in order to ensure the necessary doses for its clinical trial also in phase 3, which should begin in mid-March.
The process with Abdala, developed by the Center for Genetic Engineering and Biotechnology (CIGB), takes place at the vial factory owned by AICA Laboratories.
Abdala has already demonstrated safety and immunogenicity against Covid-19. Its phase 2 clinical trial began on February 1 at the Saturnino Lora hospital in the city of Santiago de Cuba, with about 760 volunteers.
According to Dr. Marta Ayala Ávila, director general of the CIGB, the results of the phase 1 study, which began on December 7, showed that the safety and reactogenicity profile of the immunogen was favorable for the two doses of the candidate vaccine studied.
"If the safety and immunogenicity results are corroborated, this phase 2 will be evaluated in March (the trial must be completed by March 15), and we will be preparing quickly to consider a phase 3," she said.
In total, Cuba is working on four candidate vaccines at the same time, Finlay's Soberana 01 and Soberana 02, and CIGB's Abdala and Mambisa. The latter is the only one to be administered nasally.
In this way, the Caribbean country could become the first Latin American country to have its own Covid-19 vaccines.
26/02/2021
(Translation: ALAI)
Source: Prensa Latina
https://vermelho.org.br/2021/02/25/vacina-cubana-soberana-02-pronta-para-testes-clinicos-da-fase-3/
Del mismo autor
- Cuban vaccine Soberana-02 ready for phase 3 clinical trials 26/02/2021
- Vacina cubana Soberana-02 pronta para testes clínicos da fase 3 26/02/2021
- Movimento latino-americano e caribenho pede fim do bloqueio a Cuba 17/02/2021
- EUA colocam cinco mil e seiscentos soldados na fronteira com o México 09/11/2018
- Cúpula da OTAN começa em Bruxelas sob protestos pela paz 11/07/2018
- Cadê a prova?, desafia Lula 06/07/2018
- Temer põe o Brasil à venda 15/09/2016
Pandemia
- Gabriela Ramírez Mendoza 07/02/2022
- Jyotsna Singh 06/02/2022
- Gabriela Ramírez Mendoza 06/02/2022
- Richa Chintan 10/01/2022
- Isaac Enríquez Pérez 03/01/2022
